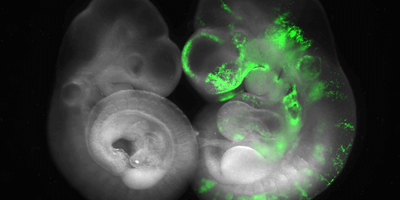
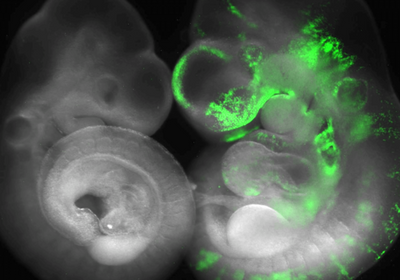
ABOVE: Researchers in Jun Wu’s lab compare normal mouse embryos (left) to horse-mouse chimeric embryos (right) to identify barriers to interspecies chimerism. Leqian Yu, UT Southwestern
“When I was in college, my mother needed a heart transplant,” said Mary Garry, a cell biologist at the University of Minnesota. Even though she was only in her fifties, Garry’s mother was told that she was too old to receive a transplant; there simply were not enough hearts available.
“My mother died in 1980,” said Garry. “The number of donor hearts that are available today is really not much more than the number that was available later on in the 1980s.”
The need for organs, especially kidneys, livers, and hearts, far outstrips availability. Today, more than 100,000 people in the United States alone are on the national transplant waiting list (1). Many will die while waiting.
As scientific understanding of stem cells, gene editing, and organism development improved, Garry felt that her career path was clear. “When the technologies became available to solve this problem, it seemed very much like the right thing to do.”
Today, Garry and her husband Dan, a transplant cardiologist, are pioneers in the field of interspecies chimera research, the study of organisms containing cells from two different species. Their team focuses on using human induced pluripotent stem cells to grow human tissues inside pigs.
Other scientists, including Jun Wu, a stem cell biologist at the University of Texas Southwestern Medical Center, are also studying chimeras with the ultimate goal of one day being able to grow enough human organs to meet the enormous need for transplants, potentially saving hundreds of thousands of lives. “Human pluripotent stem cells harbor the potential to provide an inexhaustible supply of donor cells or tissues or organs for transplantation,” Wu wrote in an email.
Monsters or miracles?
In Greek mythology, the chimera was a fire-breathing monster — part goat, part snake, part lion — that terrorized the people of Lycia before being slain by the hero Bellerophon. In biology, a chimera is much less monstrous; it is any organism that contains two or more sets of DNA. This can range from the relatively pedestrian, such as a person who received a bone marrow transplant, to creatures that seem more at home in science fiction, such as animals containing cells or tissues belonging to other species.
Lab-created interspecies chimeras are not especially new. Scientists at the ARC Institute of Animal Physiology announced their creation of sheep-goat hybrids known as geeps in 1984 (2). Early chimera research was difficult and inexact. Scientists painstakingly removed tissue from one embryo and grafted it into another embryo (3). However, advances in stem cell research in the 2000s revolutionized the field, opening up new possibilities and new applications for multispecies organism research.
In 2007, a team at Kyoto University created pluripotent stem cells from adult human somatic cells (4). Researchers began to dream of a future in which a patients’ own cells, perhaps from the blood or the skin, could be converted into these induced pluripotent stem cells and grown into whatever organ the patient needed. Not only would this provide an adequate supply of organs, but it would also eliminate the need for patients to take potentially dangerous immunosuppressant drugs; since the new organs would be made from their own cells, they wouldn’t have to worry about organ rejection.

CREDIT: CARLOS PINZON ARTEAGA, UT SOUTHWESTERN
Some researchers are attempting to use stem cells to bioengineer human organs in the lab in vitro, rather than inside another species (5). While Garry readily acknowledges the importance of multiple approaches, she said that there are important advantages to growing organs in developing animals rather than in vitro.
“We think that the developmental cues that exist in the pig will help to guide the human cells inside the porcine embryo. In the in vitro approach, there is a physical scaffold that exists, but the biological cues like the growth factors or the sheer force of blood flow or other things of that nature that are present in the living organism are missing,” she said. Scientists may not yet know enough to accurately mimic all of the developmental cues that instruct the cells to become a specific organ. “Nature knows more than we do because we can't reinvent all those things in vitro,” said Garry.
One method for growing an organ from one animal inside of a different species is blastocyst complementation. Researchers knock out a gene that drives the development of a specific organ in the host blastocyst and implant pluripotent stem cells from a donor species.
Early studies demonstrated that this technique worked, at least in some closely related species. In 2010, stem cell biologist Hiromitsu Nakauchi and his team at the University of Tokyo deleted a gene that drives pancreas formation in mouse embryos and injected rat pluripotent stem cells to fill the empty niche. The resulting mice were born with functional pancreases made of mostly rat cells (6).
Since then, Nakauchi and others have created chimeric organisms with “replacement” livers, lungs, and kidneys (7–9). Despite these successes in rodent models, translating these findings to human organs is proving to be much more challenging.
Pig possibilities
How closely animals are related seems to be an important factor in determining how easily an interspecies chimera can be created. In this sense, it might be easiest to grow human organs in closely related nonhuman primates. However, many scientists believe that logistically, pigs are the most suitable species for large-scale production of human organs: Pigs mature quickly, have large litters, and their physiologies are similar to humans in many ways (10). At 90 to 200 pounds, a miniature pig’s body size is more similar to a human’s than a typical research monkey’s; male rhesus macaques are only about 17 pounds on average, which could present difficulties when trying to grow human-sized organs (11).
Even though pigs are optimal for growing human organs in some respects, their evolutionary distance from humans creates some difficulties. During his postdoctoral research at the Salk Institute for Biological Studies, Wu explored strategies for making human stem cells more suitable for this task.

CREDIT: UT SOUTHWESTERN
By coaxing human pluripotent stem cells into an intermediate form, somewhere in between a naïve stem cell and a primed stem cell, Wu, along with other researchers at the Salk Institute, produced the first human-pig chimeric embryos in 2017 (12). Although this was a major step forward, it was a far cry from pigs with fully human organs. Researchers estimated that the embryos contained about one human cell for every 100,000 pig cells (12,13).
Deleting pig genes that drive development of specific organs, as Nakauchi did in mice and rats, can help more human stem cells grow in pig embryos, but it’s still not enough to produce a fully human organ or tissue type. The Garrys knew that the human cells needed an extra boost, so they used human cells that overexpressed BCL2, an antiapoptotic factor. By combining these boosted human cells with pig blastocysts that lacked the master regulator gene ETV2, the Garrys successfully produced a pig embryo with a fully human endothelium, the tissue that lines the vascular system, including the heart and blood vessels (14).
While endothelium transplants aren’t feasible, Garry said that this is still an important step forward. In other models, the kidney or pancreas may be made of cells from another species, but the endothelium was still made of host cells, which play a large role in organ rejection by the transplant recipient.
The endothelium is so important that “it's possible that just knocking out the vasculature with a single gene deletion — ETV2 — may be enough to make every porcine organ compatible to transplant into humans. The site of rejection is primarily the endothelium that lines the vasculature,” Garry said.
The Garrys also created pig embryos with human skeletal muscle tissue, this time deleting the p53 protein in the human cells to boost growth (15). While these studies show that growing human tissues in pigs is possible, these growth-boosting strategies aren’t necessarily appropriate for creating organs for transplant into humans, as both genetic alterations also come with an increased risk of cancer growth.
Garry’s team is currently working on understanding the relationship between the human stem cells and the pig cells of the host embryo, which may lead to strategies to increase the growth of the human cells that are more suitable for organs destined for transplantation.
“We think that the efficiency of the chimerism really comes down to immunological barriers,” she said. “So, we’re working with various other groups that are really experts in immunobiology, including David Sachs’ group at Columbia University, who are helping us to understand all these factors that present hurdles for advancement.”
Garry estimated that organs grown in pigs could be ready for trials in humans in as little as five years.
Many paths forward
Other researchers are exploring alternative strategies to increase efficiency even in evolutionarily distant animals (16,17).
Wu hopes to make progress in this area by exploring differences in chimerism between closely and more distantly related organisms. “Comparing differences of human extended pluripotent stem cells in mouse and monkey blastocysts in culture will help us understand species barriers during early development due to genomic evolution and develop better strategies to overcome these barriers to enable more robust contribution of human chimerism in evolutionarily more distant species, e.g. pigs,” wrote Wu.
To this end, Wu, along with a team of researchers from the Salk Institute for Biological Studies and the Kunming University of Science and Technology, created the first human-monkey chimeric embryos in 2021 (18).
Wu also identified cell competition between animal and human cells during development as an important factor in chimerism failure (19). “Cell competition is known to serve as a quality control mechanism to selectively remove unfit cells from a developing embryo. Human pluripotent stem cells are thus treated as unfit cells in a growing animal embryo and are targeted for elimination," wrote Wu.
By investigating the mechanisms underlying this process, Wu’s team identified ways to help human cells survive in animal embryos. When growing mouse-human embryos, they found upregulation of genes related to the NF-κB signaling pathway in the human cells. This pathway controls many different cellular functions, including response to stress and apoptosis, a type of cell death. By genetically altering this pathway, the researchers improved the survival of human cells in the mouse embryos (19).
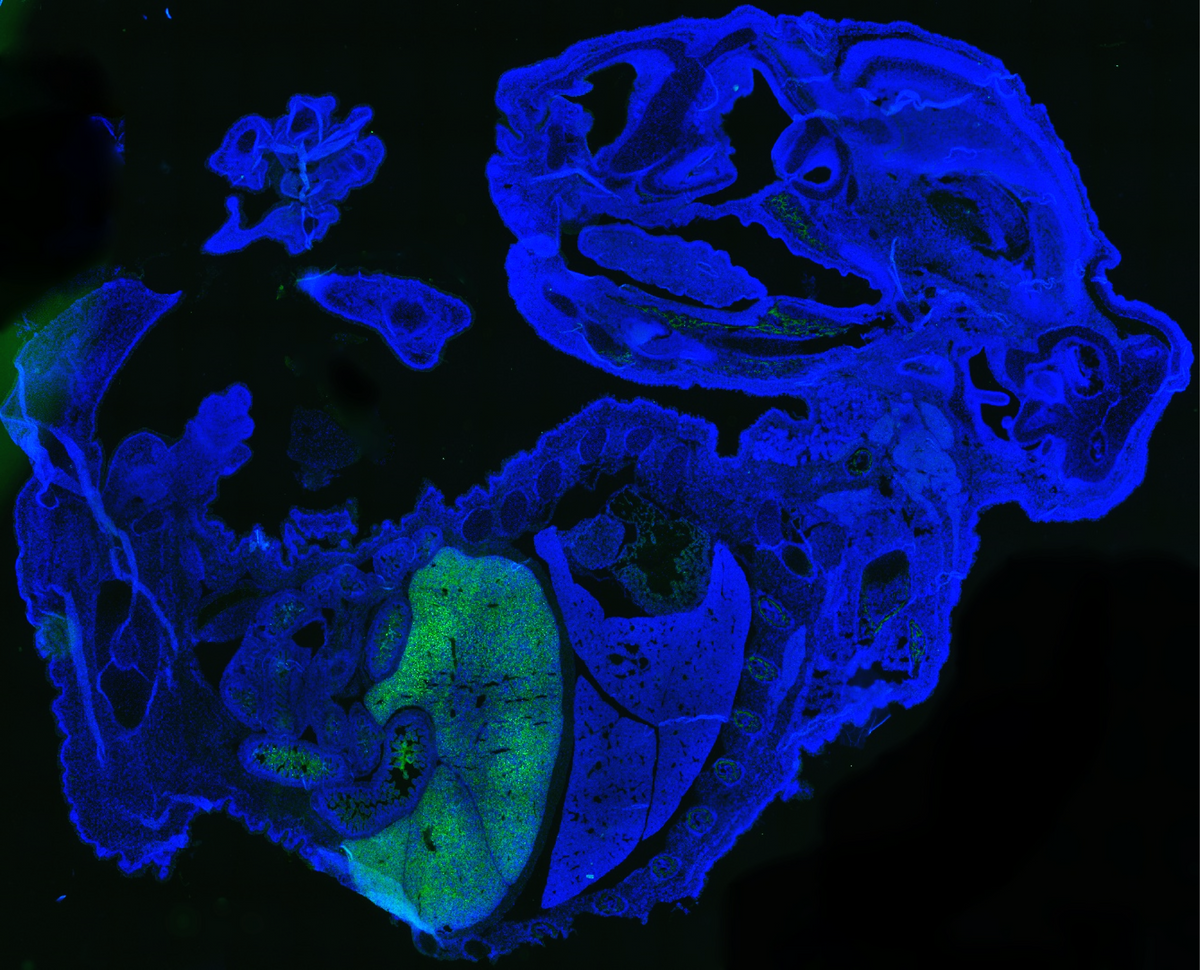
CREDIT: ZHIXING HU
Jian Feng, a stem cell researcher at the University at Buffalo, is developing another technique to encourage human cells to grow in mouse embryos. Unlike Garry and Wu, Feng’s goal is not to grow human organs, but to improve our understanding of neurodegenerative disease.
Feng was originally trained as a molecular biologist, but early on, he became interested in studying Parkinson’s disease, especially the early-onset form of the disease caused by a mutation in the PRKN gene. “I felt that I could use the full power of molecular biology to study a complex human disease,” he said.
Feng soon encountered the difficulties of studying human diseases in mice. When he knocked out the PRKN gene in the mice, they didn’t seem affected at all. So, when he read about the creation of mouse induced pluripotent stem cells, followed shortly by the generation of the human versions in the 2000s, he knew immediately that he wanted to use these human cells to study this human disease.
Studying the cells in culture wasn’t necessarily the answer though. “If we want to study human CNS problems, we have to have a circuit,” said Feng. Furthermore, the dopaminergic neurons of the substantia nigra, which degenerate in Parkinson’s disease, can’t be easily re-created in a dish. “These are very unique cells. They have extremely complicated axon arborization,” said Feng. “We needed to find a surrogate that could allow us to make these cells in vivo.”
Like other researchers, Feng has been working on strategies to improve the efficiency of human-animal chimerism. By temporarily inhibiting the signaling protein mTOR, Feng converted human pluripotent stem cells into a form that displayed improved growth when transplanted into mouse embryos; after 17 days, some of the mouse embryos had as many as four percent human cells (16).

CREDIT: SANDRA KICMAN
Eventually, Feng wants to use chimera technology to create better models of Parkinson’s disease, such as mice with human dopaminergic neurons in the substantia nigra. However, he noted that there are still many technical issues to overcome.
While creating rodents with human brain cells is a daunting task, it isn’t completely without precedent. In 2017, a group at the University of Rochester created chimeric mice with glial cells, the non-neuronal cells of the central nervous system, from induced pluripotent stem cells derived from human patients with schizophrenia (19). The glial cells developed abnormally, and the mice displayed anxiety, impaired social behavior, and disrupted sleep patterns, suggesting that glial cells likely play a role in the development of schizophrenia, and that these mice could be used as improved models for developing new therapies.
More recently in the fall of 2022, researchers at Stanford University transplanted a human stem cell derived cortical organoid into the brain of a newborn rat (20). The human neurons integrated into the rat brain; activating them sufficiently provoked certain behaviors.
While researchers still have lots of work ahead of them, they hope that one day their efforts will provide organs for transplants and a deeper understanding of neuropsychiatric diseases, saving lives and alleviating suffering around the world.
References
- Organ Donation Statistics | organdonor.gov. at <https://www.organdonor.gov/learn/organ-donation-statistics>
- Fehilly, C. B., Willadsen, S. M. & Tucker, E. M. Interspecific chimaerism between sheep and goat. Nature 307, 634–636 (1984).
- Eames, B. F. & Schneider, R. A. Quail-duck chimeras reveal spatiotemporal plasticity in molecular and histogenic programs of cranial feather development. Development 132, 1499–1509 (2005).
- Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
- Shaheen, M. F. et al. Sustained perfusion of revascularized bioengineered livers heterotopically transplanted into immunosuppressed pigs. Nat Biomed Eng 4, 437–445 (2020).
- Kobayashi, T. et al. Generation of Rat Pancreas in Mouse by Interspecific Blastocyst Injection of Pluripotent Stem Cells. Cell 142, 787–799 (2010).
- Goto, T. et al. Generation of pluripotent stem cell-derived mouse kidneys in Sall1-targeted anephric rats. Nat Commun 10, 451 (2019).
- Ruiz-Estevez, M. et al. Liver development is restored by blastocyst complementation of HHEX knockout in mice and pigs. Stem Cell Res Ther 12, 292 (2021).
- Kitahara, A. et al. Generation of Lungs by Blastocyst Complementation in Apneumic Fgf10-Deficient Mice. Cell Rep 31, 107626 (2020).
- Choe, Y., Sorensen, J., Garry, D. J. & Garry, M. G. Blastocyst complementation and interspecies chimeras in gene edited pigs. Frontiers in Cell and Developmental Biology 10, (2022).
- Rhesus macaque. Wisconsin National Primate Research Center at <https://primate.wisc.edu/primate-info-net/pin-factsheets/pin-factsheet-rhesus-macaque/>
- Wu, J. et al. Interspecies Chimerism with Mammalian Pluripotent Stem Cells. Cell 168, 473-486.e15 (2017).
- Begley, S. First human-pig chimeras created, sparking hopes for transplantable organs — and debate. STAT (2017). at <https://www.statnews.com/2017/01/26/first-chimera-human-pig/>
- Das, S. et al. Generation of human endothelium in pig embryos deficient in ETV2. Nat Biotechnol 38, 297–302 (2020).
- Humanized skeletal muscle in MYF5/MYOD/MYF6-null pig embryos | Nature Biomedical Engineering. at <https://www.nature.com/articles/s41551-021-00693-1>
- Hu, Z. et al. Transient inhibition of mTOR in human pluripotent stem cells enables robust formation of mouse-human chimeric embryos. Sci Adv 6, eaaz0298 (2020).
- Nishimura, T. et al. Generation of Functional Organs Using a Cell-Competitive Niche in Intra- and Inter-species Rodent Chimeras. Cell Stem Cell 28, 141-149.e3 (2021).
- Tan, T. et al. Chimeric contribution of human extended pluripotent stem cells to monkey embryos ex vivo. Cell 184, 2020-2032.e14 (2021).
- Zheng, C. et al. Cell competition constitutes a barrier for interspecies chimerism. Nature 592, 272–276 (2021).
- Windrem, M. S. et al. Human iPSC Glial Mouse Chimeras Reveal Glial Contributions to Schizophrenia. Cell Stem Cell 21, 195-208.e6 (2017).
- Revah, O. et al. Maturation and circuit integration of transplanted human cortical organoids. Nature 610, 319–326 (2022).

This story was originally published on Drug Discovery News, the leading news magazine for scientists in pharma and biotech.