There are millions of sensory worlds that humans cannot perceive. Fathoming what it is like to be a wild thing is beyond the limited resolution of human senses. For example, insects live in a world of smell, decoding the subtle chemical nuances of scent that waft across their antennae. In the case of locusts—the migratory swarming form of certain asocial grasshoppers—their sense of smell is intimately tied to their physical and communal transformation. Certain environmental conditions trigger locusts to molt and secrete pheromones that attract more locusts.1,2 After shedding their old body and solitary existence, billions of locusts aggregate in devastating swarms that eclipse the sun and plague humans. This dramatic body and lifestyle makeover depends on their exquisite ability to detect and differentiate subtle odors.3
Recently, Debajit Saha, an assistant professor of biomedical engineering at Michigan State University, and his team tapped into the odor-sensing circuitry of the locust brain to detect the scent signatures of human oral cancers. Saha previously used locusts for sniffing out bombs,4 making the transition to cancer detection an interesting one. “Cancer changes [cellular] metabolism and those changes are reflected in exhaled breath,” Saha said. Known as volatile organic compounds (VOCs), these unique chemical signatures are promising biomarkers of disease—if scientists can detect them. Other researchers are engineering artificial sensors—also known as electronic noses—to identify cancer, but their sensitivity, specificity, and speed are still limited compared to creatures that inhabit a sensory landscape of smell. Saha’s team is reverting to biology to harness nature’s most powerful biosensors. “Rather than back engineering a biological brain, we are forward engineering their computation,” Saha said.
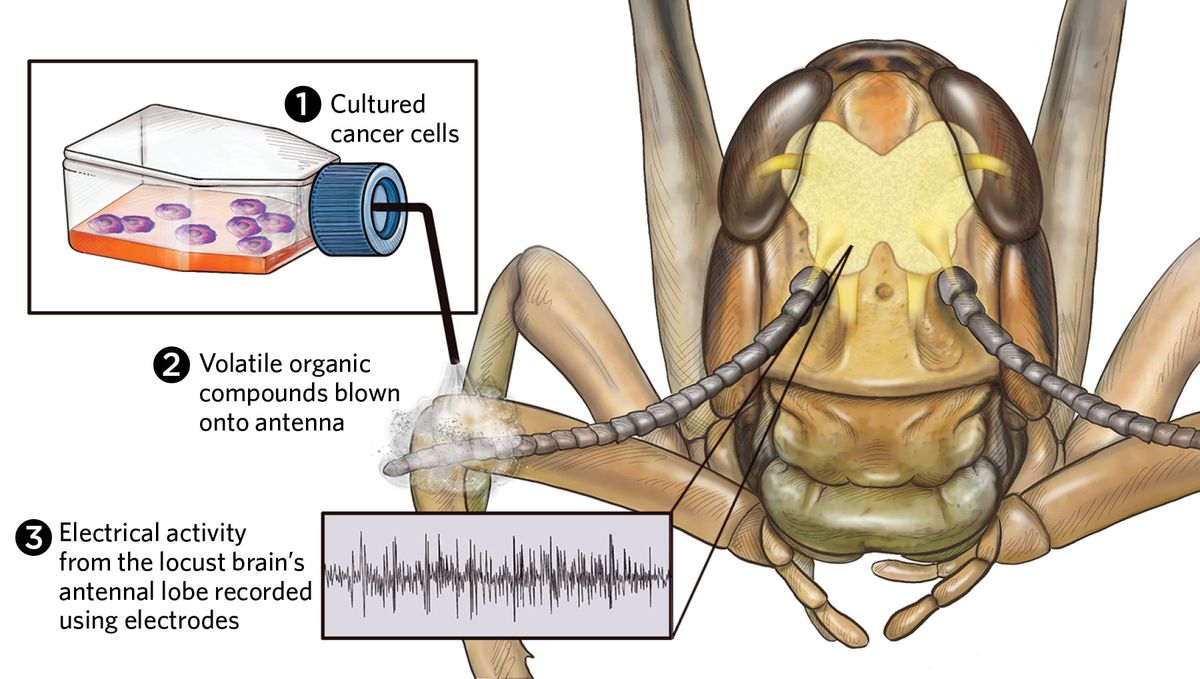
Saha’s team examined whether they could intercept the odor processing circuits in the locust brain to detect and discriminate between human oral cancer cells grow in a flask, and reported their findings in Biosensors and Bioelectronics.5 They performed brain surgery on a locust and inserted electrodes into the brain regions that process smell. Saha’s team then collected gas samples from the cell cultures of three different types of human oral cancer cells and healthy human oral cells. They wafted the gas samples—each of which contained a distinct mixture of VOCs from the corresponding cells—over the locust’s antennae and recorded the brain’s electrical activity. After pooling the results from multiple locusts, Saha’s team found that their brains produced a distinct electrical pattern for each of the different cell types. The locusts not only distinguished cancer from healthy cells but discriminated between the subtle scent fingerprints of the different oral cancers.
“It is important work that adds to our field and may one day lead to actual devices for making health diagnoses,” said Bruce Kimball, a chemical ecologist at the Monell Chemical Senses Center in Pennsylvania, who was not involved in this study. According to Kimball, any combination of a detector—sensory neurons, artificial sensors, or other analytical instruments—with a pattern analysis algorithm—biological brain, brain-mimicking chip, or machine learning—could eventually help researchers cross the finish line. Given enough fine-tuning, any such system “will likely be successful at discriminating among odors associated with most health conditions.”
Saha explained that the computational power of the locust biosensors exceeds that of current artificial sensors. Using a limited number of odor receptors, locusts have an almost unlimited capacity to encode different chemicals. “They don’t have to identify individual components, they can just take the whole mixture and create [an odor] fingerprint,” Saha said. His team plans to test breath samples from human cancer patients, which have far more complex scent signatures than cultured cells. “That will be the big test.”
After millennia of inflicting turmoil, locusts may finally redeem themselves in the aid of humankind. They remind us that our understanding of the world is only as accurate as the instruments of perception that we use.
References
- X. Guo et al., “4-Vinylanisole is an aggregation pheromone in locusts,” Nature, 584:584-88, 2020.
- H.J. Pflüger, P. Bräunig, “One hundred years of phase polymorphism research in locusts,” J Comp Physiol A Neuroethol Sens Neural Behav Physiol, 207(3):321-26, 2021.
- J.A. Cheema et al., “Insect odorant receptor-based biosensors: current status and prospects,” Biotechnol Adv, 53:107840, 2021.
- D. Saha et al., “Explosive sensing with insect-based biorobots,” Biosens Bioelectron: X, 6:100050, 2020.
- A. Farnum et al., “Harnessing insect olfactory neural circuits for detecting and discriminating human cancers,” Biosens Bioelectron, 219:114814, 2023.